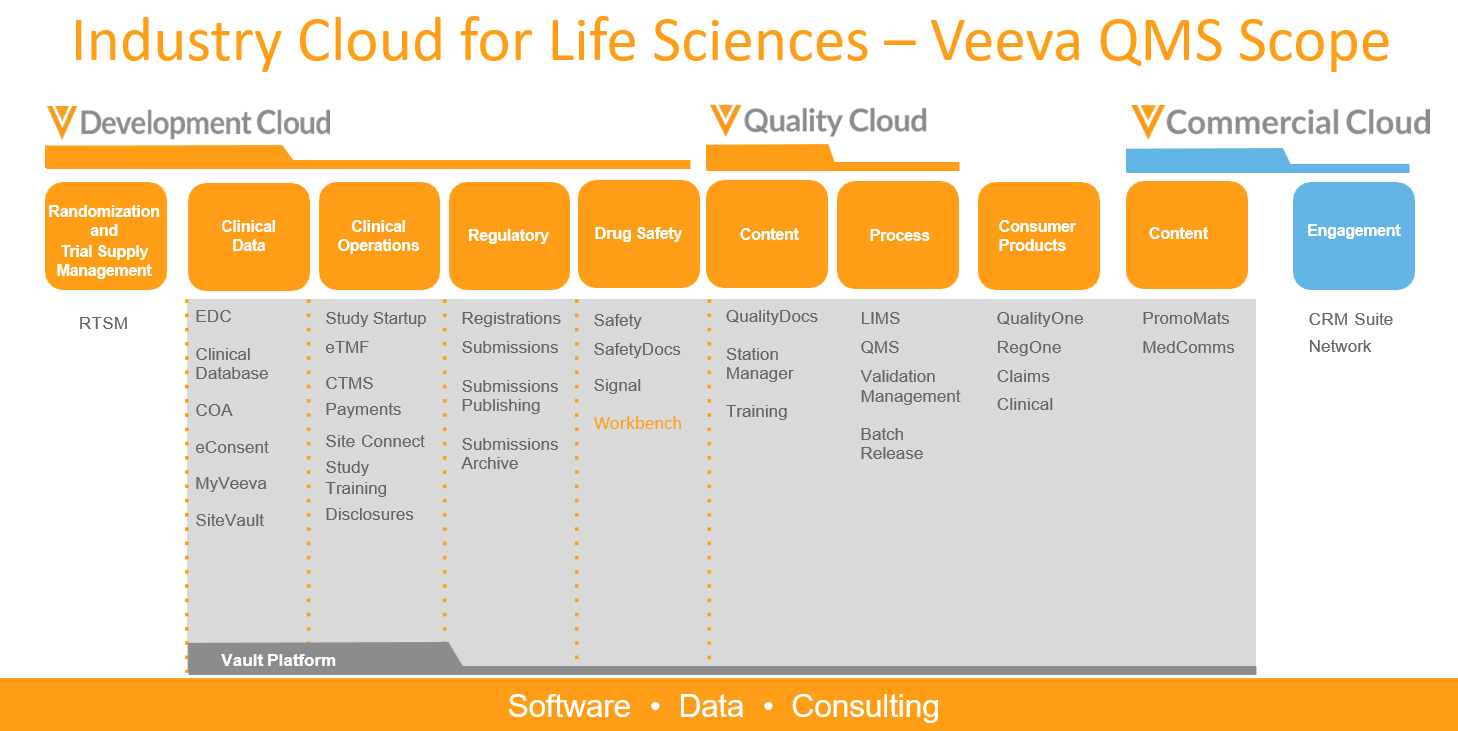
The Industry Cloud for Life Sciences
Veeva is a leader in cloud-based software for the global life sciences industry. Our solutions enable our customers to realize the benefits of a modern SaaS architecture for their most critical business needs without compromising industry-specific functionality and regulatory compliance. Our solutions span across a life sciences company, with focus on:
- Clinical - The Clinical Suite is the most comprehensive suite of clinical applications, offering EDC, coding, data management, study start-up, eTMF, CTMS and payments in a common cloud platform.
- Quality - The Vault Quality Suite is a suite of applications that enables the management of quality events and content throughout its lifecycle on a single cloud-based platform. Connecting quality processes, critical documentation and training accelerates and streamlines event identification, correction and change management.
- Regulatory - The RIM Suite provides fully integrated regulatory information management (RIM) capabilities on a single cloud-based platform, including submission document management, product registration management, health authority correspondence & commitments and submission archiving - with fully integrated IDMP capabilities.
- Drug Safety - The Safety Suite offers the first integrated suite of cloud applications on a common platform to manage the end-to-end drug safety lifecycle, from automating case intake to adverse event processing to authoring and submissions.
- Medical - The Medical Suite delivers a unified solution for scientific engagement enabling medical to better partner with healthcare to improve patient outcomes. Key customer engagement and scientific processes come together helping medical affairs transform organizational strategies.
- Sites and Patients - Sites & Patients provides a platform that connects clinical research sites with patients through features like electronic consent (eConsent), electronic patient-reported outcomes (ePRO), and the MyVeeva for Patients portal to manage trial participation and access information easily. The comprehensive product offering delivers solutions built for the unique needs of patients, sites, and sponsors.
- Commercial - The Commercial Cloud brings together multichannel engagement, commercial content, customer data and artificial intelligence to drive smarter, compliant interactions with all customers across all channels.
- Medical - Medical device and diagnostic companies are struggling to keep up with changing regulations and the growing demand for clinical data is draining resources. Vault Medical Device Suite streamlines the device and diagnostic lifecycle for greater efficiency and compliance.
- Customer Relationship Management – Vault CRM / Veeva CRM is an enterprise customer relationship management application for biopharma, consumer health, and animal health companies. It connects sales, marketing, medical, and service teams with a single customer database supporting the field, including primary care, specialty care, key accounts, retail sales, and medical science liaisons.
- Consumer Products - Veeva’s secure cloud applications help consumer goods companies (cleaning and home care, cosmetics and personal care, food and beverage, pet care, and tobacco organizations) bring high quality, safe, sustainable, and compliant products to market faster.
- RTSM - Veeva RTSM is a fast, intuitive, and complete randomization and trial supply management solution designed to simplify complex processes and expedite clinical trials. It is modular and highly configurable allowing the expert Veeva services teams to design and deliver studies with speed and accuracy. The extensive feature set allows Veeva RTSM to scale and support a spectrum of different trial design needs and complexities across all therapeutic areas. Connections with the Veeva Vault Development Cloud platform and integrations with third party solutions allow customers to seamlessly utilize Veeva RTSM in their clinical trial technology eco-system.
Additionally, Veeva provides professional services and managed services to support the implementation and on-going support of our portfolio of cloud-based applications.
Service Description Documents are available here.
System Overviews
Systems Overviews are available within the validation packages of each product suite. The overviews available to you are as follows:
- System Overview for Veeva CRM
- System Overview for Vault Platform
- System Overview for Vault CRM
- System Overview for Veeva Vault
- System Overview for Veeva CDMS
- System Overview for Veeva Safety
- System Overview for Veeva Consumer Products
- System Overview for Vault Mobile
- System Overview for Veeva Network
- System Overview for Veeva RTSM
- System Overview for Site Solutions
- System Overview for MyVeeva
- System Overview for Veeva eCOA