Vault Platform
Veeva Vault is a cloud-based information & content management platform and suite of applications which is delivered to end-users as Software as a Service, in a true multitenant SaaS model. The Vault Platform supports Veeva’s applications across its Development Cloud, Quality Cloud, Commercial Cloud, Medical, Commercial, and Consumer Product solutions. Vault is delivered and accessed through the web and hosted out of SOC 2 ISAE 3402 Type 2, ISO 27001, and ISO 9001 certified global data centers. Every major release is subject to installation qualification (IQ) and operational qualification (OQ) by Veeva. The Veeva Vault platform provides the common core functionality (e.g., security, audit trails), administrative features (e.g., working with documents) and utilities (e.g., loader and migration), across all applications.
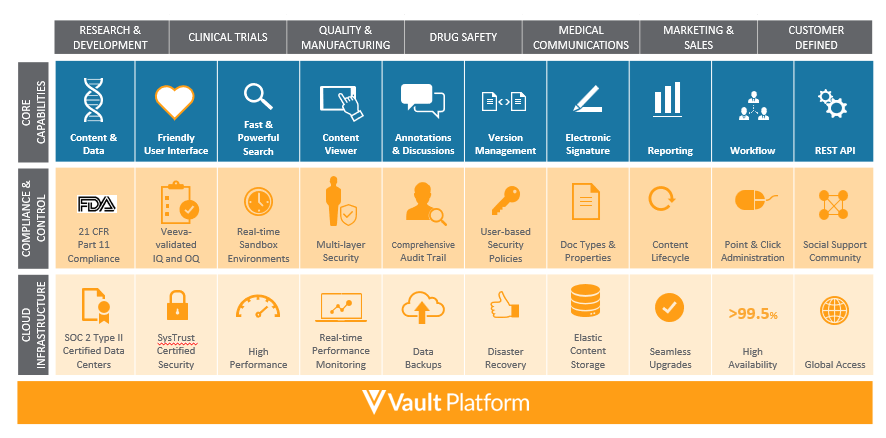
Core features of the Vault platform include:
- Dynamic Security – Address the security challenges of global enterprises with flexible authentication and authorization that easily extends across the organization and to external partners. Readily support multiple domains and fine-grain access control to functions, objects, and data with a dynamic security model.
- Electronic Signatures – Supports 21 CFR Part 11 and EU Annex 11 electronic signature capture by requiring the user to re-authenticate based on unique identification, indicating the user’s capacity and recording the signature event in the document audit trail. Electronic signatures are deployed as workflow tasks that can be included in any business process through simple, point-and-click configuration. Vault makes it easy for users to track captured electronic signatures by presenting them alongside the documents themselves, rather than forcing users to review entire audit trails to find them.
- Content Capabilities – Manage documents, videos, and images with rich capabilities including versioning, lifecycles, annotations, renditions, electronic signatures, watermarks, document generation, templates, and sharing. Rule-based configurations support complex business requirements for compliance, collaboration, and records management.
- Version Management – Vault enforces content-level version control, including both minor and major versions that can have their own security settings. Vault comes with a comparison tool that can easily show users the differences between two different versions, regardless of authoring application.
- Workflow – Automate and track business processes with configurable workflow. Provides assignment, routing, email notifications, escalation, and tracking of work items.
- Annotations and Discussions – Users can collaborate by leveraging text and image annotations in real-time directly through the built-in viewer without the need for or cost of any third-party software. The annotation functionality works across all commonly used file formats including Microsoft Office™ documents, images, and even videos. Users can respond to each other’s annotations in real-time in threaded discussions and create annotation reports, all through one common user experience.
- Open API – Easily integrate with other systems, migrate data, or automate processing using comprehensive REST APIs.
Vault Applications
Vault applications are built on top of the Vault Platform meaning all applications will utilize the platform features and functionality built in at its core, as well as unique application functional requirements.
Navigating Vault Validation Packages
Vault validation packages are divided up into two binders:
1) Vault Platform Validation
At the platform level Common functionality are tested. The common functionality of the Vault platform includes binders, compliance, document administration, document review, renditions & files, reporting & dashboards, security and viewing documents. System administration of the Vault platform includes general administration, lifecycles, object administration, workflows, working with documents, working with objects. For a complete listing of Vault Platform requirements refer to System Overview for Vault Platform (QV-37365).
2) Vault Applications Validation
Application-specific requirements are grouped and tested within the following Validation binders:
a) Veeva Applications
The following applications make up the ‘Veeva Applications’:
| Product Family | Applications |
|---|---|
| Clinical Operations Suite | - eTMF - CTMS - Site Connect - Payments - Study Startup - Disclosures - OpenData Clinical |
| Regulatory Suite | - Registrations - Submissions - Archive - Publishing |
| Quality Suite | - QualityDocs - QMS - Validation Management - Product Surveillance - Station Manager - LIMS - Training - Study Training - Batch Disposition |
| Commerical Suite | - MedComms - MedInquiry - PromoMats - MultiChannel |
For a complete listing of Vault Core application requirements refer to System Overview for Veeva Applications (QV-01873)
b) Veeva CDMS
| Product Family | Applications |
|---|---|
| Clinical Data Suite | - EDC - CDB Workbench |
For a complete listing of Vault CDMS application requirements refer to System Overview for Veeva Vault CDMS (QV-12726)
c) Veeva Safety
| Product Family | Applications |
|---|---|
| Drug Safety Suite | - Safety - SafetyDocs - Signal - Safety Workbench |
For a complete listing of Vault Safety application requirements refer to System Overview for Veeva Vault Safety (QV-16049)
d) Site Solutions
For a complete listing of Site Solutions application requirements refer to System Overview for Site Solutions
e) Veeva eCOA
For a complete listing of Veeva eCOA application requirements refer to System Overview for Veeva eCOA
f) MyVeeva
For a complete listing of MyVeeva application requirements refer to System Overview for MyVeeva
g) Veeva CP
| Product Family | Applications |
|---|---|
| Consumer Products Suite | - Claims - QualityOne - RegulatoryOne |
For a complete listing of Vault CP application requirements refer to System Overview for Veeva CP (QV-15931)
h) Vault CRM
For a complete listing of Vault CRM application requirements refer to System Overview for Vault CRM (QV-37975)